Refrigeration Systems

Steam for Energy Transfer
October 9, 2022
Standards
October 9, 2022This Homershams article begins with a brief description of the operation of a refrigeration system, and then in section 2 we examine some products that Homershams stock that are relevant to the industry.
Section 1: Basic Principles of Operation
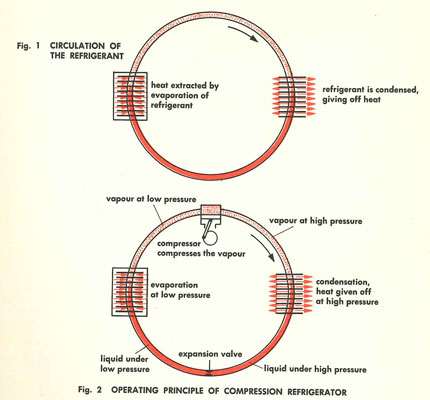
The vast majority of refrigeration systems work on the compression system. This compressive system works because of three physical properties.
- A liquid will evaporate (i.e. boil) at much lower temperatures if the pressure is low (e.g. water boils at 70 °C in the Himalayas)
- When an object changes from liquid to gas or gas to liquid, heat is absorbed or released
- The “Fist law of Thermodynamics” states that energy is never lost; just changed from different states to different states.
In a typical refrigeration system, heat from food causes a refrigerant at low pressure to evaporate (remember low pressure = low boiling point) and hence absorbs this heat. The now gaseous refrigerant is then compressed to a high pressure and then gives off its heat in the condenser.
Now one of the most misunderstood items in this system is the Thermostatic Expansion Valve (see Honeywell TEV below in section 2). The TEV is a pressure reducing valve, moving the refrigerant pressure from high to low in a controlled fashion and in response to system temperature.
TEV’s are used in commercial systems, whereas domestic refrigerators (for example) use a simple capillary tube (fixed orifice) to perform this task.
An odd, special use fridge is the gas-powered camping type. It may seem counter-intuitive to think of burning gas to make refrigeration, but it works on a similar principle to the compression fridge. In this case the refrigerant (Ammonia) is dissolved in water. When the water/ammonia mix is heated the ammonia is released as gas and hence begins the cycle as described previously.
Refrigerant GasesMany different gases may be used as refrigerants. Their rate of volatility (i.e. how quickly they will evaporate) greatly affects their relative effectiveness. A more volatile gas will more quickly evaporate, and hence remove heat. Ammonia and ether were early examples. Examining the boiling points of various refrigerants is an interesting exercise. For example the boiling point of R-23 is -82 °C! Thinking about the nature of volatile gases it’s not hard to imagine the dangers that might be associated with some of these. The tragic Tamihere cold store fire of 2008 is a timely reminder of this.
Other chemicals such as Freon many be used, but Freon is a well-known ozone destroyer, so has ecological impact. Ammonia remains a powerful and useful refrigerant, but can be unpopular with the public due to perceived danger despite the fact that it’s main public complaint is odour, which is also its greatest warning, as it’s detectable in miniscule parts-per-million (or even parts per billion) levels . One of the recommendations from the inquiry into the Tamihere cold store fire, was that “stenching agents” (smell) be added to any refrigerant gas to make their detection easier.
ASHRAE(American Society of Heating, Refrigerating and Air Conditioning Engineers) give part numbers to the many different types of refrigerant gas. A couple of examples are shown below
Glossary| Enthalpy | A thermodynamic quantity equivalent to the total heat content of a system. It is equal to the internal energy of the system plus the product of pressure and volume |
| Latent Heat of Evaporation | The amount of energy needed to be added to a system to change state from liquid to vapor without a change in the liquids temperature |
| Latent Heat of Condensation | The energy given off by a vapour condensing to a liquid |
| Superheat | The extra energy absorbed by the vapour. Remember we said that R23 boils at -82 °C? Well if we heat R23 it to 3 °C, it now contains 85 degrees of superheat available for use. |
Section 2: Products Related to Refrigeration
Click on the picture below to go to the product page

Honeywell Thermostatic Expansion Valves (previously known as Flica)

Center 382 Refrigerant Leak Detector is useful for finding leaks in a system

Teltherm 3063REF Refrigeration Pressure Gauge


